Atypical presentation of aromatic L–amino acid decarboxylase (AADC) deficiency with developmental epileptic encephalopathy
Marchese F, et al. J Pediatr Epilepsy. 2021;10(3):124–127.
Publication Date | Feb 2021
Authors | Marchese F, Faedo E, Vari MS, Bergonzini P, Iacomino M, Guerra A, Franceschetti L, Baroni A, Scudieri P, Minetti C, Striano P.
Citation | J Pediatr Epilepsy. 2021;10(3):124–127.
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0041-1723768
Aromatic L–amino acid decarboxylase (AADC) metabolises key neurotransmitters, and AADC deficiency can lead to reduced levels of dopamine, serotonin, epinephrine and norepinephrine in the central nervous system (CNS),1,2 typically resulting in a variety of motor, autonomic and behavioural symptoms. Seizures may occur over the disease course, but epilepsy is rarely associated with the condition and developmental epileptic encephalopathy (DEE) is uncommon.1
A group of researchers in Italy have published a case report of a patient with a clinical presentation of DEE, who was later diagnosed with AADC deficiency.1
The patient was a male; the second child of Moroccan consanguineous parents. There was no reported significant medical history, including neurodevelopmental disorders or epilepsy, in either parent or the patient’s 6–year–old sister. The patient was born at 39 weeks following a normal pregnancy and uncomplicated delivery. At birth, there was no evidence of CNS trauma or pathology.1
At the age of 6 months, he presented with intractable flexion and extension spasms, mainly of the upper limbs, which occurred daily at awakening. Magnetic resonance imaging (MRI) was normal and intracranial electroencephalography (EEG) showed multifocal spikes and high–voltage discharges over the posterior head regions. A number of plasma amino acid levels were outside of normal values and DEE was diagnosed.1
No clinical benefit was achieved with vigabatrin (the frequency of spasms increased to >30 per day). Add–on therapy with nitrazepam, levetiracetam and carbamazepine were also ineffective.1
At 23 months of age, a 4:1 ratio ketogenic diet was initiated with levetiracetam and nitrazepam therapy, and a reduction in spasm frequency and intensity occurred within 2 months. A repeat EEG showed reduced epileptiform discharges. The patient was able to sit unaided and use some two–word phrases. However, brief focal seizures continued to occur on a daily basis. Dystonic movements associated with these included head deviation and limb stiffening. Severe dysphagia at 5 years of age was resolved by cessation of the ketogenic diet.1
At 8 years of age, with worsening seizures, the patient was referred to the specialist centre for paediatric neurology and muscular diseases in Genoa, Italy. Seizures were different in character from the previously experienced intractable spasms and were now associated with head drop, lower limb hypotonia and abduction of the upper limbs, occurring in both wakefulness and sleep. The patient’s parents also reported isolated oculogyric crises, abnormal sweating and disturbed sleep patterns before the patient was 5 years old.1
Video EEG, repeat brain MRI and full physical and neurological examinations were undertaken at this time; the results are presented in Figure 1.1
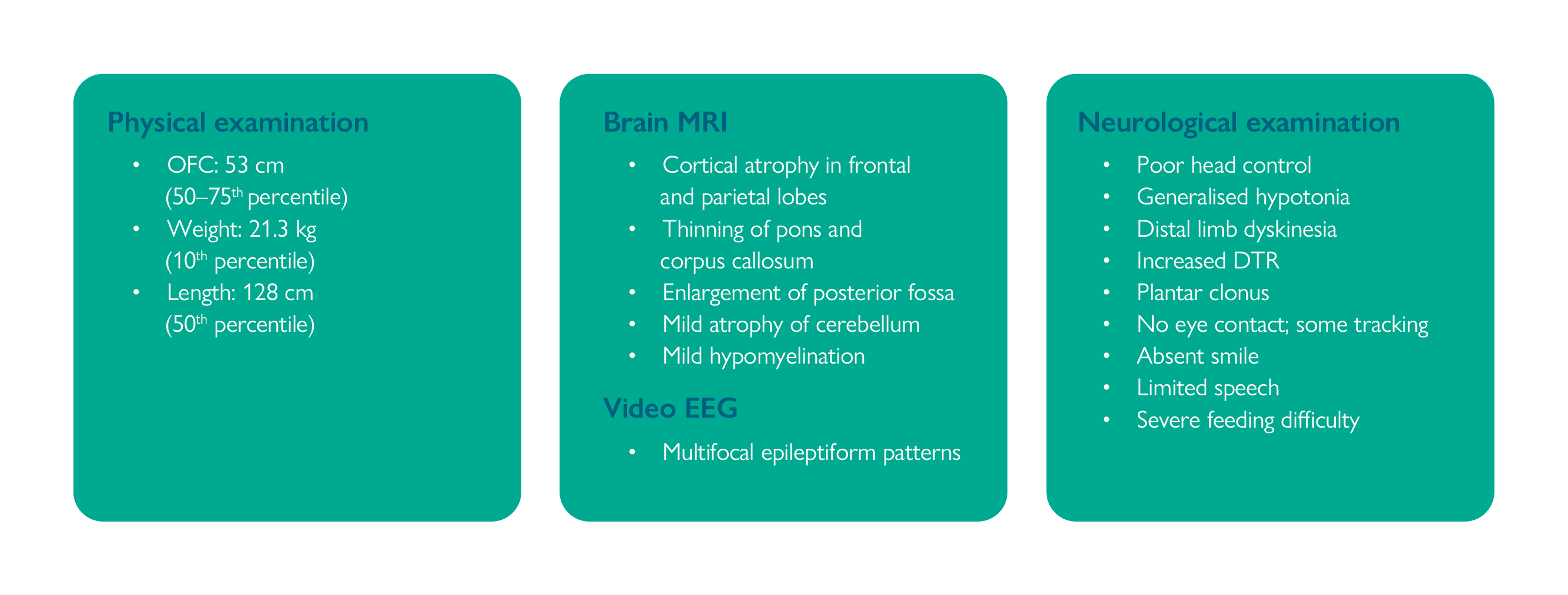
DTR, deep tendon reflexes; EEG, electroencephalography; MRI, magnetic resonance imaging; OFC, occipital–frontal circumference.
To rule out deficiencies of glucose transporter 1 (GLUT1) and syntaxin–binding protein 1 (STXBP1), sequencing assays were carried out to analyse the SLC2A1 and STXBP1 genes, respectively. No abnormalities were detected, and sequencing of a number of genes known to be associated with epilepsy was also unremarkable. No evidence of glycosylation defects was found on serum sialotransferrin screening.1
Subsequent whole exome sequencing (WES) revealed a homozygous single nucleotide variant (c.121C>A; p.Leu41Met) mutation in DDC, the gene that codes for AADC. The patient’s parents refused a confirmatory cerebrospinal fluid metabolites panel and a trial of therapy with pyridoxine.1
Concluding, the researchers noted that tonic-clonic or focal seizures are only occasionally reported in patients with AADC deficiency. However, this case study strengthens a possible link between DDC mutations and an electroclinical phenotype consistent with DEE, though further research will be needed to establish this.1
Severe DEE with infantile spasms is heterogeneous in aetiology and has been linked to a number of genetic abnormalities. In this case study, the underlying cause of the patient’s symptoms was initially elusive – assays did not reveal abnormalities in genes known to be associated with a DEE phenotype. However, on WES, a mutation in DDC was confirmed leading to a diagnosis of AADC deficiency.1
The exact incidence of AADC deficiency is unknown – patients are often misdiagnosed as clinical presentation can be similar to other neurological conditions, such as epilepsy. An accurate diagnosis is important in AADC deficiency, especially as therapeutic interventions are available. Dry blood spot testing for 3–O–methyldopa (3–OMD) levels and WES are becoming more accessible to help in the early diagnosis of the condition.1
- Marchese F, et al. J Pediatr Epilepsy. 2021;10(3):124–127.
- Wassenberg T, et al. Orphanet J Rare Dis. 2017;2:12.